An Overview of Multiple Sclerosis
MS affects 2.8 million people worldwide, over 80% of which have varying degrees of disability. MS research has shown that the disease not only impacts physical abilities, but also mental health and personal independence.
Patients experience a severe decline in emotional quality of life, suffering from depression or heavy emotional burden; some even have suicidal thoughts. (8)
Despite the urgency of early treatment to slow disease progression and disability, diagnostic delays remain a major problem, affecting more than 50% of patients with MS. (19)
Some patients are misdiagnosed by neurologists (19) not giving them the chance to start the appropriate therapy on time.
An early diagnosis means that early treatment initiation can start to preserve brain tissue and optimize brain health. This can maximize the chances of altering the disease course before further relapses or disability progression occurs. (5)
Causes of Diagnosis Delays in Multiple Sclerosis
A diagnosis of MS relies on the integration of clinical, imaging, and laboratory tests. This is summarized in the current iteration of the McDonald criteria (20), whereby diagnosis is based on the principles of dissemination in space (DIS) and dissemination in time (DIT) of clinical attacks and/or central nervous system (CNS) lesions and the exclusion of other diseases mimicking MS. (14)
In simple terms, this means finding MS lesions in different areas of the CNS and demonstrating that they occurred at different times (6), as well as excluding disease that present similar symptoms (12) or may present a similar appearance on magnetic resonance imaging (MRI). (1)
It has been shown that several factors, both disease-related and local, can contribute to the diagnostic delay. In particular, the difficulty of recognizing typical MS disease features, both in images and symptoms, and local factors such as access to MRI. (14)
It is now clear that MRI is crucial for the diagnosis of MS; however, it is not generally very specific, often requiring a diagnosis of exclusion, meaning that other diseases must be excluded before a diagnosis of MS can be confirmed. (17)
In short, MS is a complex condition, difficult to diagnose and easily confused with other diseases. Although the diagnosis integrates clinical, imaging and laboratory tests, none of these are highly specific for MS. The main cause of diagnostic problems is often the misinterpretation of MRI scans. (13)
As a result, the importance of identifying more specific MRI biomarkers for MS is evident.
Improving MS Diagnosis with the Central Vein Sign (CVS)
In this ongoing purpose to improve MS diagnosis and rule out MS-mimicking disorders, a biomarker has recently shown great promise: the central vein sign (CVS). (17)
The CVS is a radiological finding on neuroimaging, indicating the presence of a central vein within a white matter lesion, which is characteristic of MS. (4)
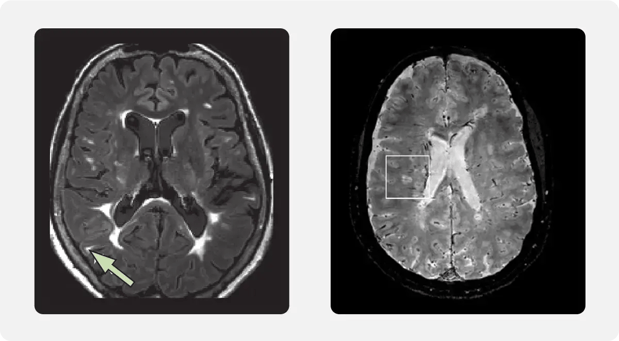
Left image: Hyperintense lesion near the cortex; source: Filippi et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines, Lancet Neurol 2016:15(3). Right Image: Image of how some MRI sequences can show lesions and CNS vessels simultaneously; source: Gill et al. Emerging imaging and liquid biomarkers in multiple sclerosis, Eur J Immunol 2023:53(8)
What started 13 years ago as an hypothesis by Nikos Evangelou’s team at Nottingham (18) has grown into a recent advancement for the early diagnosis of MS. CVS in MS lesions first emerged in 2011 and was proposed as a potential MS biomarker (7), then in 2016 the North American Imaging in MS Cooperative issued a consensus statement on its presence (15). From 2017 onwards, the scientific community turned its attention to discovering the potential of CVS, both to understand its role in the disease and to refine detection techniques.
In 2021, the CAVS-MS trial (Central Vein Sign: a Diagnostic Biomarker in Multiple Sclerosis) was born, a pioneering effort to validate CVS as a diagnostic biomarker.Clinical trials like CAVS-MS are pivotal in advancing multiple sclerosis research, and QMENTA has played a key role in managing the data infrastructure supporting this groundbreaking research.(11)
The CAVS-MS Trial: Advanced Imaging Workflow for CVS Detection
CAVS-MS clinical trial was initiated as a collaborative effort to support the MS clinical trials landscape. This imaging trial highlights the potential of innovation and technology, as well as QMENTA’s commitment, to advancing the understanding of MS and so improving patient outcomes.
The trial sees the QMENTA Imaging Hub serve as a centralized repository and infrastructure for all participating parties, 2 institutions and 9 sites: Cleveland Clinic - collaborating with Dr Daniel Ontaneda, Cedars-Sinai in collaboration with Dr Pascal Sati, and then a number of other participating sites: Johns Hopkins University, University of Pennsylvania, St. Michael's Hospital, University of Colorado, University of South California, University of Texas at Austin, University of Vermont, Washington University and Yale University.
At QMENTA, we manage the implementation of the CAVS-MS workflow, which has led to significant improvements in the efficiency and accuracy of clinical data management for CVS research in MS.
QMENTA’s workflow implementation includes:
- Convert DICOM files into NIfTI format
- Analyze FLAIR and FLAIR-post images from different MRI scans
- Evaluate image quality with scores from 3 reviewers
- Segment white matter hyperintensity (WMH) lesion and include confidence maps for each class
- Remove false positives in the lesion detection
- Provide a tool for manually editing WMH lesions
- Compare the central vein sign (CVS) before and after using contrast
By automating protocol adherence rules, defining 16 distinct user roles and digitizing and implementing electronic reporting forms (eCRF) with dynamic logic, we simplify and ensure the entire process from data entry to data analysis is seamless and robust.
Our query management system ensures data integrity by flagging inconsistencies for a timely resolution, while our ongoing support throughout the project enables its smooth operation.
This whole process not only optimizes data quality, but also allows researchers to focus on what really matters, advancing CVS research.
CVS-MS: From Emerging Indicator to Diagnostic Biomarker
In 2023, the use of advanced techniques such as FLAIR* allowed researchers to refine the detection of CVS and it was recommended for the first time to be included in the McDonald criteria. (12)
Now, in 2024, the CVS is being considered for inclusion in the diagnostic criteria for MS, representing a potential breakthrough in multiple sclerosis research and diagnosis...as a diagnostic biomarker has been confirmed, with the recent announcement of its incorporation into McDonald's diagnostic criteria (9).

Proposal to Include CVS in McDonald Diagnostic Criteria for MS
CVS has emerged as a very promising diagnostic tool for MS. In fact, CVS was found in 73% of lesions in patients with MS, showing a 95% of sensitivity and 92% of specificity.
A cut-off value of 40% of lesions presenting CVS has been demonstrated (3); in other words, if more than 40% of white matter brain lesions show CVS, the likelihood to diagnose MS is very high. The diagnostic accuracy of CVS is reliable with a sensitivity and specificity of 90% and 89%. (3)
In essence, CVS turns out to be highly specific and sensitive for typical MS lesions and as such is of particular value in differentiating MS from other conditions on MRI. (3)
MRI at 3T and higher field strengths are sufficiently clear to be practically useful, whereas lower field magnets (1.5T) demonstrate significant degradation in detection accuracy. (3)
In addition to diagnostic performance, CVS also has practical advantages. Unlike other neuroimaging markers, it is visible to the naked eye with appropriate sequences, and, being a binary marker, simplifies the diagnostic process. This simplicity is particularly important in both typical and atypical MS clinical presentations, where early and accurate diagnosis can lead to a timely therapeutic intervention. (10)
In conclusion, including CVS as a diagnostic criterion has the potential to significantly improve specificity over currently employed methods and makes it an important tool for diagnosis and post-diagnostic management of MS (10). Higher specificity means less room for error and uncertainty, which increases the possibility of having accurate diagnoses at the right time, therefore being able to start the right therapies in a timely manner. Clinical trials like CAVS-MS and ongoing neuroimaging advancements continue to push the boundaries of what’s possible, providing new tools to reduce diagnostic delays and improve patient care.
References
- Aliaga et al. (2014), MRI mimics of multiple sclerosis. Handb Clin Neurol (122)291-316.
- Al-Louzi et al. (2022), Central Vein Sign Profile of Newly Developing Lesions in Multiple Sclerosis: A 3-Year Longitudinal Study. Neurology(R) neuroimmunology & neuroinflammation, 9(2).
- Castellaro et al. (2020), The Use of the Central Vein Sign in the Diagnosis of Multiple Sclerosis: A Systematic Review and Meta-analysis. Diagnostics (Basel) 10(12).
- Gill et al. (2023), Emerging imaging and liquid biomarkers in multiple sclerosis. Eur J Immunol 53(8).
- Giovannoni et al. (2016), Brain health: Time matters in multiple sclerosis. Multiple Sclerosis and Related Disorders, 9(1).
- Kaisey et al. (2024), Multiple Sclerosis Diagnostic Delay and Misdiagnosis. Neurol Clin 42(1).
- Kau et al. (2013) The “central vein sign”: is there a place for susceptibility weighted imaging in possible multiple sclerosis?. Eur Radiol (23)1956–1962.
- Kołtuniuk et al. (2023), The quality of life in patients with multiple sclerosis – Association with depressive symptoms and physical disability: A prospective and observational study. Frontiers in Psychology, 6(13).
- Montalban X. 2024 Revisions of the McDonald Criteria. Presented at ECTRIMS Congress; September 18-20, 2024; Copenhagen, Denmark. Scientific Session 1: New diagnostic criteria.
Oh et al. (2020), Detection of central vein should be part of MS diagnostic criteria - Commentary. Mult Scler 26(4).
- Ontaneda et al. (2021), Central vein sign: A diagnostic biomarker in multiple sclerosis (CAVS-MS) study protocol for a prospective multicenter trial. NeuroImage. Clinical (32)102834.
- Ontaneda et al. (2023), Incorporating the Central Vein Sign Into the Diagnostic Criteria for Multiple Sclerosis. JAMA Neurol. 80(7).
- Patti et al. (2022), Factors driving delayed time to multiple sclerosis diagnosis: Results from a population-based study. Multiple Sclerosis and Related Disorders, 57(103361).
- Patt et al. (2022), Factors driving delayed time to multiple sclerosis diagnosis: Results from a population-based study. Mult Scler Relat Disord 57(103361).
- Sati et al. (2016), The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol (12)714–722.
- Solomon et al. (2023), Differential diagnosis of suspected multiple sclerosis: an updated consensus approach. Lancet Neurol 22(8).
- Sparacia et al. (2018), Multiple sclerosis: High prevalence of the ‘central vein’ sign in white matter lesions on susceptibility-weighted images. The Neuroradiology Journal, 31(4).
- Tallantyre et al. (2011), Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology, 76(6).
- Ther et al. (2023), Diagnostic delay of multiple sclerosis: Prevalence, determinants, and consequences. Multiple Sclerosis, 29(11-12).
- Thompson et al. (2018), Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2).


